

Welcome to the wonderful world of Cavazaque – a mysterious and versatile ingredient that is taking the culinary scene by storm! If you’re looking to amp...


Key Takeaways Understanding chronic pain and its various treatment options is essential for effective management. Lifestyle factors, including diet, exercise, and sleep, can significantly influence chronic...
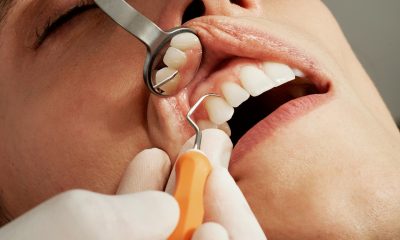
Explore the long-term health benefits of dental implants and the latest advancements, including metal-free options. Find out why Kirkland, WA is becoming a hub for top-notch dental implant...


Table of Contents Benefits of guava for Sensualty Guava leaf tea recipe Guava Juice Recipe Related Guava is a tropical fruit with a very particular sweet...

Table of Contents Benefits of the Yakult drink 1.- Yakult fight bacteria 2.- Yakult for digestion 3.- Immune system 4.- Yakult eliminate toxins 5.- Flatulence 6.-...


Table of Contents The long history of Lipton tea Health benefits of drinking Lipton green tea 1.- Benefits of lipton tea for digestion 2.- Lipton tea for...


Table of Contents What happens when you eat okra every day? Okra sexually What does okra do to a woman? What are the side effects of...


Table of Contents Overview Nutritional value of cashew leaves Health Benefits Of Cashew Leaves 1. Benefits of cashew leaves for cholesterol 2. Benefits of cashew leaves...

Everyone is looking to lose weight these days, but most people miss the one key to just how easy it really is: eating more fiber! While...


Table of Contents How did Chaz Bono achieve his weight loss goal? Chaz Bono weight loss diet How did Chaz Bono lose 60 pounds? What has...